- HOME
- Business
- Drug discovery business
- Microparticle technology
Microparticle technology
Microparticle formulations are suitable for the sustained release effect of compounds because the polymer releases the drug at a constant concentration and continues to travel throughout the body. Thus, microparticle-based pharmaceutical products are desired. However, the formulation technology is challenging.
We are developing a sustained release microparticle formulation. We have received the transfer of technology related to the formulation design of sustained release injection from Okada DDS Laboratory Co., Ltd (https://okada-dds.com/company.html) and have built a comprehensive system from feasibility testing to formulation design and good manufacturing practice (GMP) manufacturing.
Feasibility study of the formulation design for long-term release injection formulation
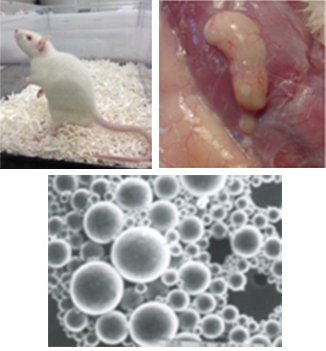
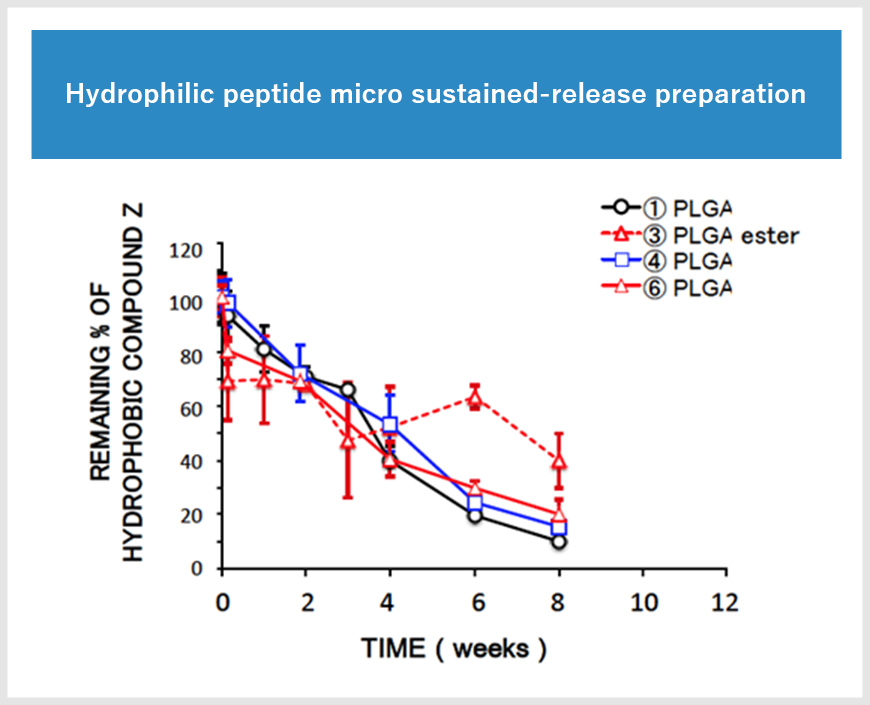
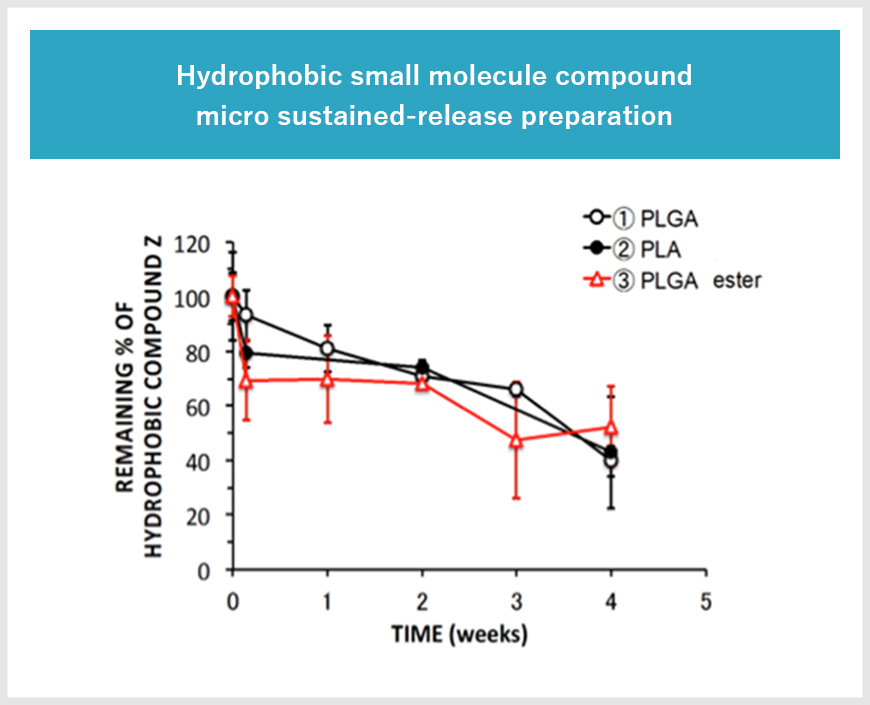
Long-term release preparations are essential to reduce the burden on patients by reducing the number of doses and improving the quality of life (QOL). We selected the polymer most compatible with the drug from more than 15 types of polymers with different molecular weights. The final formulation design according to the desired sustained release period was achieved.
Approximately 20 types of polymers, including GMP compatible carboxylic acid-terminated and ester-terminated PLGA and PLA are available from domestic and overseas manufacturers (GMP compatible).
Joint research and development
Preparation adjustment with SENTAN Pharma's prescription ~ In vivo release evaluation ~ Final preparation design
Scale-up / GMP manufacturing is conducted through a comprehensive system from research to GMP manufacturing through a technical arrangement with a domestic chief marketing officer (CMO) company.
Advantages of SENTAN Pharma